The easily performed experiment in catalysis of heating a mixture of Potassium Chlorate and Manganese Dioxide was made. The catalytic changes observed were as follows (representing by O and O the Oxygen atoms belonging respectively to Potassium Chlorate and Manganese Dioxide)
B. THE COMBINATION OF HYDROGEN AND OXYGEN TO
FORM WATER, IN THE PRESENCE OF PLATINUM
In this case there is little chemical evidence of the formation of intermediate compounds. The action is represented 2H,+O, -2 H,O.
The Platinum seems to act as an agent to produce the right conditions rather than to take much part in the action itself.
This is borne out in the occult investigation, where the change of the energy conditions is described by Mr. Leadbeater as a compression. The substances taking part in the reaction become denser or are compressed together, and in this condition the union of the two gases, Hydrogen and Oxygen, takes place.
It will be seen that in the notes the 'compression' is mentioned, but it is further stated that " The platinum does not do more than draw the Hydrogen atoms round it" To the chemist this suggests the surface film produced on the surface of metals.
C. J. Do the
bars of the Platinum revolve more rapidly round each axis ?
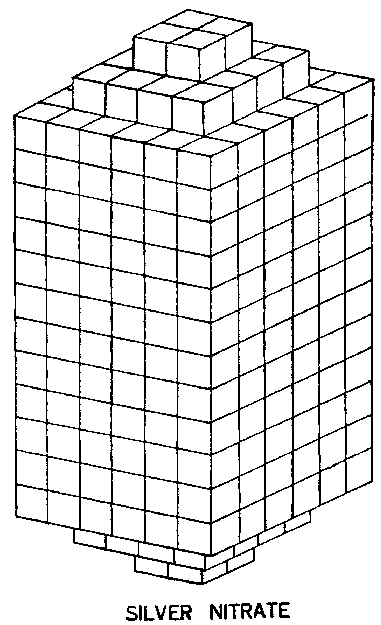
FIG. 214. A SILVER NITRATE CRYSTAL

FIG. 215. THE EFFECT OF LIGHT ON
SILVER NITRATE
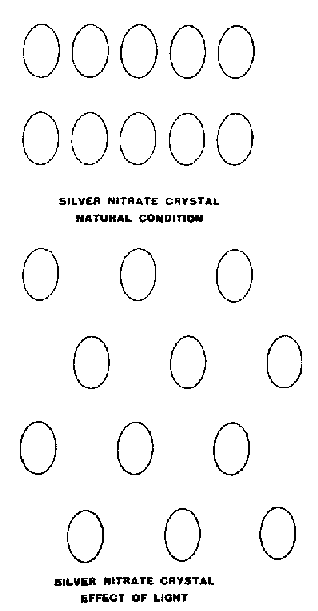
Observation showed that the Silver Nitrate compound existed first in groups of 1,296 molecules, which then broke up into groups of 432 when subject to light
Fig. 214 shows the crystal of Silver Nitrate, its shape being that of a double cube tapering at both ends. When light impinges on it, it is broken up into three blocks, each of 432 molecules. In these smaller blocks, also the ends are pushed out so that the blocks taper at each end
Fig. 215 illustrates the effect produced by light on the arrangement of the molecules. In the normal crystal the molecules are in rows. Light alters their position so that they are as in the diagram. The alternate molecules step back. Evidently the light is absorbed and not reflected.
CALCITE AND ARAGONITE
The constitution of these two forms of CaCO5 appears identical, but in one the three Oxygen atoms stand upright at right angles to the paper, and in the other they radiate horizontally as drawn in Figure 172, page 276.
THE DIAMOND
When examined clairvoyantly it was seen that the structure of the Diamond was somewhat difficult to grasp. There was clearly a unit of Diamond, and its shape was a triakis octahedron. Fig. 216. But how was the large mass of Carbon atoms built up to make the Diamond? Each Carbon atom is an octahedron in outline; each is composed of eight funnels, four positive and four negative. Obviously in any form of packing, funnels of like electrical quality must not come mouth to. mouth, as they will then repel each other.
One especial difficulty in mapping out the structure of the Diamond was due to the fact that in reality there is no rigid octahedral shape visible in the outline of a Carbon atom. Certainly its eight funnels radiate to the eight surfaces of an octahedron; but the octahedral shape is more an appearance than a reality. Fig. 217 shows four of these funnels. The funnel is a temporary effect, being in fact the rotational field made as groups of Anu revolve. In their revolutions, they push back the circumambient matter of the plane next above, making thus a temporary shell or field of activity.
In the packing of Carbon to make the Diamond, any two funnels of opposite electrical quality, from two adjacent Carbon atoms, interlock. The two rotational fields overlap, and the cigarshaped bodies of one funnel enter among the interstices of the similar bodies in the funnel opposite to it. Fig. 218 is an attempt to show this interlocking. This unusual interlocking may perhaps be the reason why the Diamond crystal is so very hard.
The simplest way to describe the Diamond, whose general appearance is shown by Fig. 219, is to narrate how the octahedrons are assembled, in the making of the model First, five Carbon atoms are grouped, as in Fig. 220. Funnels of opposite electrical quality hold each other rigidly. These five Carbon atoms, in this formation, form the Carbon molecular unit for the building of the Diamond Fig. 221 shows the same unit, with its Maltese cross, as seen from the back.
Taking now 25 of these units, we place them in rows of five, making thus a square. Similarly we assemble 16 units to make a smaller square, 9 more to make a square smaller still, and finally 4 to make the smallest square. We now make a pyramid of four sides; its base will be of 25 units, then next above 16, 9 and 4. The top of the pyramid is one unit of five Carbon atoms.
Here we quote the words of the investigator as he describes what he sees.
" Now build in imagination another pyramid exactly like the first, and one would expect, by putting them together base to base, to have the complete molecule. But it is not so simple as that. They are applied base to base, but they are, as it were, bolted together by the insertion of additional Carbon atoms. Turn the pyramid upside down, and you will see quite a pretty pattern of 25 Maltese crosses. Fig. 222. Take any four of these crosses, and you will see in the middle of the group of four a depression, a square hole. In the reversed base of 25 units there are 16 of these holes, and before we set the bases together we must put a single carbon atom in each of the 16 holes of one of the bases. The 16 atoms will project like spikes, but when we apply the two bases, we shall find that these projections will exactly fit into the depressions which come opposite to them, and will lock the two pyramids together most efficiently. Is this also part of the explanation of the extreme hardness of the diamond ?
" There is yet another peculiarity. The 16 blue and black holes (in the diagram) are arranged in four lines of four. Produce those lines in each case to the edge of the base of the reversed pyramid, and we find another additional Carbon atom fixed there as a bolt; also, one extra at each corner of the base. We will mark the holes for these (they are really only half-holes) green in our diagram, and there will be twenty of them altogether. The Carbon atoms which fill these green exterior holes project at the sides of
FIG. 217. FOUR CARBON FUNNELS.
FIG 218. CARBON INTERLOCKING
FIG. 214. A CRYSTAL OF DIAMOND
FIG. 220. FIVE CARBON ATOMS
FIG. 221.
VIEW
SHOWING
MALTESE CROSS
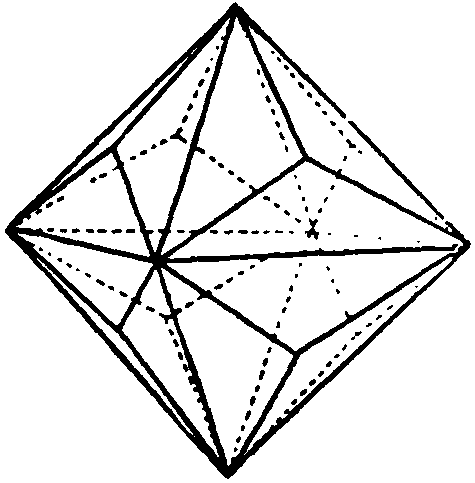

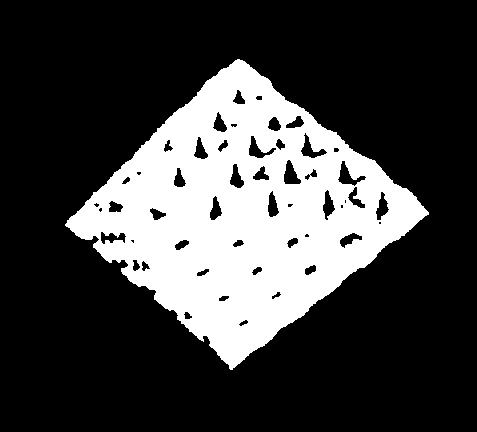

 Fig. 222. The Stricture of the Diamond
Fig. 222. The Stricture of the Diamond
 CATALYSIS,
CRYSTALLIZATION 339
CATALYSIS,
CRYSTALLIZATION 339
the base of the pyramid, and make a serrated edge. Has this anything to do with the remarkable cutting power of the diamond?
" It seems noteworthy that the molecule stands always on the point of one of its pyramids, like a buoy floating in the water. In building the two pyramids, the units (of five Carbon atoms) always stand upright on their crosses; consequently it follows that when we reverse one of those pyramids to apply their bases, all the units in both of them are pointing away from the centre of the molecule. The little grey lozenges on the diagram are orifices, through which the background can be seen.
" I find it extraordinarily difficult to describe the thing so that there can be no mistake about it; I feel as though there must be some other way of looking at it which would make it all perfectly simple, but I cannot just get that point of view; perhaps someone else will. You have probably no idea of the trouble it has cost to analyze this molecule; it seems different from anything I have tackled before.
" There is still one more peculiarity, which however is not represented in the model The whole molecule is, as I have said, a flattened octahedron, and of course its eight sides are triangles. But in the middle of each of these eight sides-or rather aver the middle of it-hovers a single floating Carbon atom, floating out at right angles to the face of the triangle, pointing straight away from its centre. Its bottom point is almost touching the central point of the side, but not quite. I suppose that we could make it appear to float in its place by some ingenious attachment of thin wire, or possibly a long pin. Tiny as this Carbon atom is, it produces a curious effect We know how each chemical atom makes a shape for itself by pushing back surrounding matter-a shape which is really illusory, like the octahedron for the Carbon atom, whose sides are actually the mouths of funnels. Without those eight floaters, the shape of this Diamond molecule would be a flattened octahedron; but each of them raises the centre of its triangle very slightly, so that lines run from that centre to each angle of the triangle, dividing it into three very flat triangles, and so making the molecule a twenty-four sided figure, the triakis octahedron. The lines, of course, run from the apex of the floating atom."
Therefore
in two pyramids .... .... 550 Atoms
In 16 blue holes .... .... 16 _
In 20 green half-holes .... ....
20 _
Floating atoms .... .... 8 _
Total ....
594 Atoms
340 OCCULT
CHEMISTRY
GRAPHITE
It is well known that Graphite, which is dark grey and lustrous, is also composed of Carbon atoms. While the Diamond is hard, Graphite is soft and friable. Obviously the packing in Graphite must be quite different. Each octahedron in the figure is a Carbon atom of eight funnels; the difference in the electrical quality of the funnels is shown by light faces of the octahedron for positive, and dark faces for negative funnels.
The arrangement of the octahedrons in Graphite is such that, in each ring of six, a positive funnel is linked to a negative, and vice versa. Two layers of Carbon atoms in this formation can exist linked one over another, as the under surface of each layer is exactly the reverse electrically of the upper surface, and so two contacting surfaces readily link. .
This open-work lace-pattern arrangement of Carbon atoms accounts for the peculiarities in Graphite of darkness and of lustre. When light falls from the top, most of it enters in, and therefore when looked at from that particular angle, Graphite is dark. When light falls from the side, the absorbing spaces are much smaller in comparison, and a great deal of the light is thrown back, but not all of it, as in the case of the Diamond The friability of Graphite is easily understood when we note its arrangement into layers, as described above.
WITH the information revealed in Occult Chemistry a great expansion of our knowledge of Chemistry lies in front of us. It is just because this expansion is inevitable, that our clairvoyant investigators have toiled patiently for thirty years. They have claimed no recognition from chemists and physicists, because truth accepted or rejected is truth still, and any fact of nature seen and stated dearly will sooner or later be woven into the whole fabric of truth. The fact that this generation of scientists hardly knows anything at all of an extraordinary work of research extending for thirty years matters little, when we contemplate the long vistas of scientific investigation which the imagination sees awaiting mankind.
I desire to express my deep sense of obligation to the following members of the Theosophical Society, who gave their voluntary services in drawing various diagrams
1. S. V. Kanakasabha Pillai, Executive Engineer, Retired, Public Works Department, Government of Madras; 2. S. Narayanamurty, Retired Draughtsman, Superintending Engineers Office, Bezwada ; 3. J. Lippincott, Ojai, California, U.S.A., who, during a few weeks' stay at Adyar Headquarters, drew the large diagram of the Periodic Table, given as the frontispiece; 4. Arthur N. Relton, England; 5. Harry S. Banks, New Zealand; 6. F. L. Kunz, U.S.A., who 25 years ago gave assistance in the construction of the model of the four Lemniscates depicting the Periodic Table. Fig. 14. After millimetre squared paper had been mounted on a number of rods he mapped out the position of the elements, a work redone by Mr. Relton.
I must express my hearty thanks also to Mr. V. John, owner and manager of Klein and Peyerl, who for thirty years have provided me with the necessary blocks for this and other works. This firm has put at my disposal all their talent in the way of draughtsmen, etc. and for Occult Chemistry, Mr. John has himself given much advice and assistance for the blocks.
| ATOMIC | ELEMENT | ANALYSIS OF THE STRUCTURE OF | NO. OF |
| NO. | THE ELEMENTS | ANU | |
| 1 | Hydrogen | (2H3'-I-H3)+(3H3) | lg |
| Adyarium | 4H3+4Ad6 or Ad12-f-Ad24 | 36 | |
| Occultism | 2H3+Ad24+Oc15-t-Oc9 | 54 | |
| 2 | Helium | 2H3+(2H3'-f-H3)-i-(3H3)+2Ad24 | 72 |
| 3 | Lithium | (4Li4)+Li63+8Ad6 | 127 |
| 4 | Beryllium | Be4-E-4 (4Be10) | 164 |
| 5 | Boron | (4B5)-E-6 [4(2H3)+Ad6] | 200 |
| 6 | Carbon | 4+4 (C27-f-C26) | 216 |
| 7 | Nitrogen | N110+N63t2N24+2N20 | 261 |
| 8 | Oxygen | (55N2+5.0.7)+(55N2+5.0.7') | 290 |
| 9 | Fluorine | 2N110+8 (2Be4-I-H3'-+-Li4) | 340 |
| 10 | Neon | Ne120+6 [Ne22+(3Li4)+(2H3)] | 360 |
| Meta-Neon | Ne120-f-6 [Ne22+mNe15-i-L7+H3] | 402 | |
| 11 | Sodium | Na14+2Na10+24Na16 | 418 |
| 12 | Magnesium | 4 [3 (3Mg12)] | 432 |
| 13 | Aluminium | 6 [AL9'-l-8A1.9] | 486 |
| 14 | Silicon | 8 [B5-f-4Si15] | 520 |
| 15 . | Phosphorus | 6 [(B5-f-3N6-h3P9)-f-(Li4+3Be4+3P9)] | 558 |
| 16 | Sulphur | 4 [3 (3S16)] | 576 |
| 17 | Chlorine | CL19+2Na10-E24CL25 | 639 |
| Proto-Argon | Nel20+6 [N63+Ne22+L7] | 672 | |
| 18 | Argon | Ne120+6 [N63-f-Ne22+Ar14] | 714 |
| Meta-Argon | Ne120+6 [N63-f-Ne22+mNe15+mAr6] | 756 | |
| 19 | Potassium | (N110-+6Li4)-f-9Li63 | 701 |
| 20 | Calcium | Ca80+4Ca160 or Ca80-f-4 [Ca45-f-Ca70~-Ca45] | 720 |
| 21 | Scandium | (4B5-t Be4)-f-3 [N110+4 (2H3)+Ad6]-f- | |
| 3 [N63 +2N24+B5] | 792 | ||
| 22 | Titanium | (Ne120+8) t 12Ti14+4 (Ti88+.C27+C26+ 1) | 864 |
| 23 | Vanadium | (L7+4B5)-t-3 [N110+N20+4 (2H3)-t-Ad6]+ | |
| 3 [N63+2N24+N20+N6j | 918 | ||
| 24 | Chromium | (8N6+8Ad6)+4 (Ca160+2Cr25) | 936 |
| 25 | Manganese | N110+ 14Li63 | 992 |
| 26 | Iron | 14 [2Fe14+Fe16+Fe281 | 1008 |
| 27 | Cobalt | 14 [2Fe14+Fe16+2Co11+Co81 | 1036 |
| 28 | Nickel | 14 [2Fe14+Fe16+2Co11+Ni10] | 1064 |
| 29 | Copper | C1.19+2 [2Be4+2Ad6]+24 [Cl?5+2B5+Cu10] | 1139 |
| 30 | Zinc | (Zn18)+4 [3(3516)]+4 [4Zn20+3Zn18'+CulOJ | 1170 |
| 31 | Gallium | 6 [(Gal+3Ga15+3Ga20)+(B5+3Ga13+3Ga18)] | 1260 |
| 32 | Germanium | (Be4+2Ad24)+8 [4Ge39J | 1300 |
| 33 | Arsenic | 6 [AL9'+8 (2N9+AL9)] | 1350 |
| 34 | Selenium | Zn18 +4 [3 (3Se10+3Se10+3N2)+Se153] | 1422 |
OCCULT CHEMISTRY 343
|
|
ELEMENT |
|
|
|
|
|
|
|
| 35 | Bromine | CL19+2 (Be4+2H3+2N2)+24 (CL25+3GeI1) | 1439 |
| 36 | Krypton | Ne12U+6 [N63+N110+Ne22+mNe15+Ar14] | 1464 |
| Meta-Krypton | Ne120+6 [N63+N1I0+Ne22+Ne22+Ar14] | 1506 | |
| 37 | Rubidium | (3N110)+16 [Li63 +RbI2] | 1530 |
| 38 | Strontium | (Sr96)+4 (2Ca160+2Sr24) | 1568 |
| 39 | Yttrium | (Ad24+Yt16)+6 [N63+N110+Yt44+(4Yt8+2Ad6)] | 1606 |
| 40 | Zirconium | (Ne120+8)+12Lr36+4 (Zr212+C27+C26+1) | 1624 |
| 41 | Niobium | (2Ad24+N9)+6 [N63+N110+yt44+Nb60] | 1719 |
| 42 | Molybdenum | (N2 f Sr96) +4 (2CaI60+2Mo46) | 1746 |
| 43 | Masurium | (3N110)+16 [Li63+ Ma29 (a or b)] | 1802 |
| 44 | Ruthenium | 14 [2Fe16+2Fe14+2Ru17+2Ru19] | 1$48 |
| 45 | Rhodium | 14 [2Fe16+2Fe14+2Rh20+2Rh17] | 1876 |
| 46 | Palladium | 14 [2Rh17+2Pd15+2Pd17+2Pd19] | 1904 |
| 47 | Silver | CL19+2(m-Ne5+2H3+2N2)+24(CL25+3Ge11+Ag21) | 1945 |
| 48 | Cadmium | Cd48+4[3 (3Se10+3Zn18'+4Zn20)] | 2016 |
| 49 | Indium | 3 [2 (In16+3Ga15+3Ga20)+(In14+3Ga13+3Ga18)]+ | |
| 3 [(InI6+3Ga15+3Ga20)+2 (In14+3Ga13+3Ga18)] | 2052 | ||
| 50 | Tin | Ne120 +8 (4Ge39)+6 Sn126 | 2124 |
| 51 | Antimony | 3 [2Sb128+Sb113]+3 [2Sb113+Sbl28] | 2169 |
| 52 | Tellurium | (Cd48+3)+4 [3 (3Se10+3Te21+4Te22)] | 2223 |
| 53 | Iodine | CL19 +2 (3Be4+2H3)+24 (CL25+3Ge11+S.L7) | 2287 |
| 54 | Xenon | Ne120+6 [Xe15+Xe14+N63+2N110+Ne22+ | |
| mNe15+Ar14] | 2298 | ||
| Meta-Xenon | Ne120+6 [2mXe18+N63+2N110 Ne22+mNe15+ | ||
| Ar14] | 2340 | ||
| 55 | Caesium | (4N110) + 16 [Li63 + 2Ma29a1 | 2376 |
| 56 | Barium | (L7+Sr96)+4[2 Ca160+2Mo46+Ha33+Li63b+Ba80] | 2455 |
| 57 | Lanthanum | (Ne120+L7)+3 [N63+N110+Mo46+Ca70+ | |
| Yt44+Nb60]-I-3 [N63+N110+Ca45+Ca70-f- | |||
| Yt44-I-Nb60] | 2482 | ||
| 58 | Cerium | Ce667+4Zr212+4 [Ca160-I-Ce36-I-C27+C26] | 2511 |
| 59 | Praeseodymium Ce667-f-6 [Pr33+N63-I-N110+Yt44+Nb60] | 2527 | |
| 60 | Neodymium | Ce667-i-4 [2Ca160-1-2Mo46+Nd65] | 2575 |
| 61 | Illinium | (4N110)+8 (2Li63-I-I1.9)-E-8 [2Li63+I1.14] | 2640 |
| X | 14 [3X30-I-3X28-hX15] | 2646 | |
| Y | 14 [3X30+2Y29-X28-X15] | 2674 | |
| Z | 14 [3X30-3231+Cu10] | 2702 | |
| 62 | Samarium | (2Sm84+4Sm66) +2Sm101-E-24 (C1.25-1-4Ge11-f-Ag21) | 2794 |
| 63 | Europium | Eu59+4 [3 (3Se10-l-3Eu26+4Eu31)] | 2843 |
| 64 | Gadolinium | Ne120-)-3 [2Sb128-I-Sb113-I-(Ca45+2NZ4)] | _ |
| +3 [Sb128+2Sb113+(Ca45+Mo11+2N24)J | 2880 | ||
344 OCCULT CHEMISTRY
| ATOMIC | ELEMENT | ANALYSIS OF THE STRUCTURE OF |
|
| NO. | ~ THE ELEMENT |
|
|
| 65 | Terbium | Ne12U+8 (4Ge39+2Mo46+L7)+6Sn126 |
|
| 66 | Dysprosium | Ne120-f-3 [2Sb128+Sb113+(Ca45+2Mo11+2N24)] | |
|
|
|
||
| 67 | Holmium | Ho220+4 [3 (3Se10+3Eu26+4Eu31)] |
|
| 68 | Erbium | (CL19+3Sm84+6Sm66)+2Sm101+24 [CL25+4Ge11+ | |
| Ag21] |
|
||
| Kalon | Ne120+6 [Xe15+Xe14+2N63+2N110+2Ne22+ | ||
| 2mNe15+2Ar14+Ka12] |
|
||
| Meta-Kalon | Ne120+6 [2mXe18+2N63+2N110+2Ne22+ | ||
|
|
|
||
| 69 | Thulium | (4N110)-X16 [2Li63-t-Tm40] |
|
| 70 | Ytterbium | Yb651 +4 [2Ca160+2Mo46+(Ca160+Yb48)] |
|
| 71 | Lutetium | Lu819+6 [N63+N110+Lu53+Ca70+Lu36+Nb60] |
|
| 72 | Hafnium | Hf747 -I-4 [Zr212+4Hf36]-f-4 [Ca160-bCe36-f- | |
|
|
|
||
| 73 | Tantalum | Lu819-I-6 [N63+N110-f-Ta63+Ca70-1-Yt44-f-Nb60] |
|
| 74 | Tungsten | Lu819+4 [2Ca160+2Mo46+Ca160+Yb48] |
|
| 75 | Rhenium | (4N110)-f-16 [2Li63-I-Re571 |
|
| 76 | Osmium | 14 [4X30 + 3231 + Os32] |
|
| 77 | Iridium | 14 [4X30-f-2Ir27+2Ir26+Ag21] |
|
| 78 | Platinum | 14 [4X30+21r26+2X28+Ag21] |
|
| Isotope | 14 [4X30 + 2Ir27 + 2X28 + A9211 |
|
|
| 79 | Gold | Au864+2 (Sm101+2Au38)+24 [C125+4Ge11+Fe28] |
|
| 80 | Mercury | Au864+4 [3 (3Se10-f-3CL19+4Te22)+Se153] |
|
| 81 | Thallium | T1.687-f-3 [2Sb128-f.Sb113-f-(Ca45+T1.44+2N24)] | |
| +3 [Sb128+2Sb113+(Ca45+TL44+2N24)] |
|
||
| 82 | Lead | T1.687+4 [Ca160*Mo46+4Sn35+Pb31] | |
| +4 [Ca160+Mo46+4Ge39+Pb21] |
|
||
| 83 | Bismuth | T1.687+3 [2Sb128+Sb113+(Ca45+Mo46+2N24)] | |
| +3 [Sb128-I-2Sb113-1-Ti88-b(Ga20-f-4Zr13)J |
|
||
| 84 | Polonium | Po405+4 [3 (3Po17t3Po33+4Po33')] |
|
| 85 | 85 | Au864+2 (Sm101+2Au38)t24 [CL25+2+4.85.15+ | |
| Fe28] |
|
||
| 86 | Radon | Ne120+6 [Xe15+Xe14+2N63+3N110+3mNe22+ | |
|
|
|
||
| Meta-Radon | Ne120+6 [Xe15+Xe14+2N63+3N110+3mNe22+ | ||
| 3mNe15-t-3Ar14+L7+mRd7] |
|
||
| 87 | 87 | (5N110)-X16 [3Li63+87271 |
|
| 88 | Radium | Lu819+4 [3Ca160+3Mo46]+4 [3Li63+Cu10] |
|
| 89 | Actinium | Lu819+3 [N63+N110+Mo46+Ca160+Yt44+ | |
| Nb60]+3 [Zr212+Sb128+Ac116]-f-8Li63 |
|
OCCULT CHEMISTRY 345
|
|
ELEMENT |
|
|
|
|
|
|
|
| 90 | Thorium | Lu819+4 [Zr212+Sb128+Ac116] | |
| -+4 [(Ca160+Mo46-f-2Li63-f-C27+C26+1)) |
|
||
| 91 | Proto- | Lu819+3 [N63+N110+Mo46+Ca160+Yt44-f-Nb60] | |
| Actinium | +3 [Zr212-1-Sb128-E.Ac116-bPa29] | ||
| + 8Li63 |
|
||
| 92 | Uranium | Lu819+4 [3Ca160+3Mo46]+4 [3Li63+ | |
| U66+Ur19] |
|
This Table includes a comparison between the scientific and the occult atomic weights. The scientific atomic weights were calculated from the International list of atomic weights 1949, where O 16.00 and H =1.008. The final decision as to the names of elements Nos. 43, 61, 85 and 87 was made too late to be used in this book.
|
|
|
|||||
| ATOMIC ELEMENT | SYMBOL | NUMBER |
|
|
|
|
| NO. | OF ANU HYDROGEN |
|
|
|||
|
|
SCALE | |||||
| 1 | Hydrogen | H | 18 | 1.00 | 1.00 | Ovoid |
| Adyarium | Ad | 36 | 2.00 | - . | Ovoid | |
| Occultum | Oc | 54 | 3.00 | Ovoid | ||
| 2 | Helium | He | 72 | 4.00 | 3.97 | Star |
| 3 | Lithium | Li | 127 | 7.06 | 6.89 | Spikes |
| 4 | Beryllium | Be | 164 | 9.11 | 8.94 | Tetrahedron |
| 5 | Boron | B | 200 | 11.11 | 10.73 | Cube |
| 6 | Carbon | C | 216 | 12.00 | 11.91 | Octahedron |
| 7 | Nitrogen | N | 261 | 14.50 | 13.90 | Ovoid |
| 8 | Oxygen | 0 | 290 |
|
15.87 | Ovoid |
| 9 | Fluorine | F | 340 | 18.88 | 18.85 | Spikes |
| 10 | Neon | Ne | 360 | 20.00 | 20.02 | Star |
| Meta-Neon | mNe | 402 | 22.33 | |||
| Il | Sodium | Na | 418 | 23.22 | 22.81 | Dumb-bell |
| 12 | Magnesium | Mg | 432 | 24.00 | 24.13 | Tetrahedron |
| 13 | Aluminium | A1 | 486 | 27.00 | 26.76 | Cube |
| 14 | Silicon | Si | 520 | 28.88 | 27.84 | Octahedron |
| 15 | Phosphorus | P | 558 | 31.00 | 30.73 | Cube |
| 16 | Sulphur | S | 576 | 32.00 | 31.81 | Tetrahedron |
| 17 | Chlorine | Cl | 639 | 35.50 | 35.17 | Dumb-bell |
| Meta-Chlorine | mCl | 667 | 37.06 | - | ||
| 18 | Argon | Ar | 714 | 39.66 | 39.68 | Star |
| Meta-Argon | mAr | 756 | 42.00 | |||
| Proto-Argon | pAr | 672 | 37.33 | |||
| 19 | Potassium | K | 701 | 38.94 | 38.79 | Spikes |
| 20 | Calcium | Ca | 720 | 40.00 | 39.76 | Tetrahedron |
| 21 | Scandium | Sc | 792 | 44.00 | 44.74 | Cube |
| 22 | Titanium | Ti | 864 | 48.00 | 47.52 | Octahedron |
| 23 | Vanadium | V | 918 | 51.00 | 50.55 | Cube |
| 24 | Chromium | Cr | 936 | 52.00 | 51.60 | Tetrahedron |
| 25 | Manganese | Mn | 992 | 55.11 | 54.50 | Spikes |
| 26 | Iron | Fe | 1008 | 56.00 | 55.41 | Bars |
| 27 | Cobalt | Co | 1036 | 57.55 | 58.47 | Bars |
| 28 | Nickel | Ni | 1064 | 59.11 | 58.52 | Bars |
OCCULT CHEMISTRY 347
|
|
|
|||||
|
|
ELEMENT | SYMBOL | NUMBER WEIGHT |
WEIGHT |
|
|
|
|
OF ANU |
|
|
|
||
|
|
|
|||||
|
|
Copper | Cu | 1139 | 6328 |
|
Dumb-bell |
|
|
Zinc | Zn | 1170 | 65.00 |
|
Tetrahedron |
|
|
Gallium | Ga | 1260 | 70.00 |
|
Cube |
|
|
Germanium | Ge | 1300 | 72.22 |
|
Octahedron |
|
|
Arsenic | As | 1350 | 75.00 |
|
Cube |
|
|
Selenium | Se | 1422 | 79.00 |
|
Tetrahedron |
|
|
Bromine | Br | 1439 | 79.94 |
|
Dumb-bell |
|
|
Krypton | Kr | 1464 | 81.33 |
|
Star |
| Meta-Krypton | mKr | 1506 | 83.66 | |||
|
|
Rubidium | Rb | 1530 | 85.00 |
|
Spikes |
|
|
Strontium - | Sr | 1568 | 87.11 |
|
Tetrahedron |
|
|
Yttrium | Yt | 1606 | 89.22 |
|
Cube |
|
|
Zirconium | Zr | 1624 | 9022 |
|
Octahedron |
|
|
Niobium | Nb | 1719 | 95.50 |
|
Cube |
|
|
Molybdenum | Mo | 1746 | 97.00 |
|
Tetrahedron |
|
|
Masurium | Ma | 1802 | 100.11 |
|
Spikes |
|
|
Ruthenium | Ru | 1848 | 102.66 |
|
Bars |
|
|
Rhodium | Rh | 1876 | 10422 |
|
Bars |
|
|
Palladium | Pd | 1904 | 105.77 |
|
Bars |
|
|
Silver | Ag | 1945 | 108.06 |
|
Dumb-bell |
|
|
Cadmium | Cd | 2016 | 112.00 |
|
Tetrahedron |
|
|
Indium | In | 2052 | 114.00 |
|
Cube |
|
|
Tin | Sn | 2124 | 118.00 |
|
Octahedron |
|
|
Antimony | Sb | 2169 | 120.50 |
|
Cube |
|
|
Tellurium | Te | 2223 | 123.50 |
|
Tetrahedron |
|
|
Iodine | 1 | 2287 | 127.06 |
|
Dumb-bell |
|
|
Zenon | Xe | 2298 | 127.66 |
|
Star |
| Meta-Xenon | MXe | 2340 | 130.00 | ,. | ||
|
|
Caesium | Cs | 2376 | 132.00 |
|
Spikes |
|
|
Barium | Ba | 2455 | 136.39 |
|
Tetrahedron |
|
|
Lanthanum | La | 2482 | 137.88 |
|
Cube |
|
|
Cerium | Ce | 2511 | 139.50 |
|
Octahedron |
|
|
Praeseodynium | Pr | 2527 | 140.39 |
|
Cube |
|
|
Neodymium | Nd | 2575 | 143.06 |
|
Tetrahedron |
|
|
Illinium | 11 | 2640 | 146.66 |
|
Spikes |
| Meta-Illinium | 2736 | 152.00 | ., | |||
| X Interperiodic | 2646 | 147.00 | Bars - | |||
| Y ,. | 2674 | 148.55 | Bars - | |||
| Z .. | 2702 | 15022 | Bars | |||
348 OCCULT CHEMISTRY
|
|
|
|||||
|
|
ELEMENT | SYMBOL |
|
|
WEIGHT |
EXTERNAL |
|
|
OF ANU HYDROGEN | HYDROGEN FORM | ||||
|
|
SCALE | |||||
| Isotope Z |
|
|
Bars | |||
|
|
Samarium |
|
|
|
1492 | Dumb-bell |
|
|
Europium |
|
|
|
150.8 | Tetrahedron |
|
|
Gadolinium |
|
|
|
155.7 | Cube |
|
|
Terbium |
|
|
|
158.0 | Octahedron |
|
|
Dysprosium |
|
|
|
1612 | Cube |
|
|
Holmium | Ho |
|
|
163.6 | Tetrahedron |
|
|
Erbium |
|
|
|
165.9 | Dumb-bell |
| Kalon |
|
|
Star | |||
| Meta-Kalon |
|
|
„ | |||
|
|
Thulium | TM |
|
|
168.1 | Spikes |
|
|
Ytterbium | Yb |
|
|
171.7 | Tetrahedron |
|
|
Lutetium | Lu |
|
|
173.6 | Cube |
|
|
Hafnium | Hf |
|
|
1772 | Octahedron |
|
|
Tantalum | Ta |
|
|
1795 | Cube |
|
|
Tungsten | W |
|
|
1825 | Tetrahedron |
|
|
Rhenium | Re |
|
|
184.8 | Spikes |
|
|
Osmium | Os |
|
|
188.7 | Bars |
|
|
Iridium | Ir |
|
|
191.6 | Bars |
|
|
Platinum A | Pt |
|
|
193.7 | Bars |
| 11 B |
|
|
„ | |||
|
|
Gold | Au |
|
|
195.6 | Dumb-bell |
|
|
Mercury A | Hg |
|
|
199.1 | Tetrahedron |
| B |
|
|
„ | |||
|
|
Thallium | T1 |
|
|
202.8 | Cube |
|
|
Lead | Pb |
|
|
205.6 | Octahedron |
|
|
Bismuth | Bi |
|
|
207.6 | Cube |
|
|
Polonium | Po |
|
|
208.3 | Tetrahedron |
|
|
Astatine |
|
|
|
208.3 | Dumb-bell |
|
|
Radon |
|
|
|
2202 | Star |
| Meta-Radon |
|
|
„ | |||
|
|
Francium | Fr |
|
|
2212 | Spikes |
|
|
Radium | Ra |
|
|
224.3 | Tetrahedron |
|
|
Actinium | Ac |
|
|
2252 | Cube |
|
|
Thorium | Th |
|
|
230.3 | Octahedron |
|
|
Proto-actinum | Pa |
|
|
2292 | Cube |
|
|
Uranium |
|
|
2362 | Tetrahedron | |